Organic Nomenclature - Alkenes and Alkynes
Other modules in this series:
Special thanks to Vicki Winterton for providing the outline for this learning module.
You may test your knowledge of knowledge of alkane nomenclature prior to studying this module, by using the Interactive Quiz on Alkanes
Introduction to Alkenes and Alkynes
There are two other types of basic hydrocarbons: alkenes and alkynes. Alkenes contain less hydrogen, carbon for carbon, than the alkanes. To account for this difference, the carbon atoms are joined by a double bond. The carbon-carbon double bond is the distinguishing feature of the alkene structure.
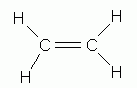
ethene
Alkynes contain an even smaller proportion of hydrogen than the alkenes. To account for this difference, the carbon atoms share three pairs of electrons . that is, they are joined by a triple bond. The carbon-carbon triple bond is the distinguishing feature of the alkyne structure.
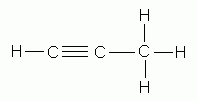
propyne
Rules for Naming Alkenes
- Select as the parent structure the longest continuous chain that contains the carbon-carbon double bond; then consider the compound to have been derived from this structure by replacement of hydrogen by various alkyl groups. The parent structure is known as ethane, propene, butene, pentene, and so on, depending upon the number of carbon atoms . each name is derived by changing the ending "-ane" of the corresponding alkane name to "-ene".
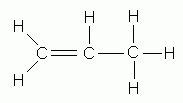
propene
- If the parent chain is longer than three carbons, indicate by a number the position of the double bond in the parent chain. Although the double bond involves two carbon atoms designate its position by the number of the first doubly-bonded carbon encountered when numbering from the end of the chain nearest the double bond.
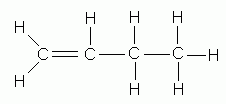 1-butene |
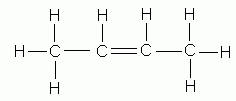 2-butene |
- Indicate by numbers the positions of the alkyl groups attached to the parent chain.
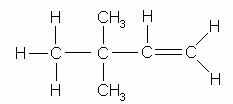 3,3-dimethyl-1-butene |
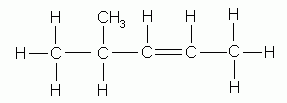 4-methyl-2-pentene |
Rules for Naming Alkynes
The alkynes can also be named by the IUPAC system. The rules are exactly the same as for the naming of alkenes except that the ending "-yne" replaces "-ene". The parent structure is the longest continuous chain that contains the triple bond, and the positions both of the substituents and of the triple bond are indicated by numbers. The triple bond is given the number of the first triply-bonded carbon encountered, starting from the end of the chain nearest the triple bond.
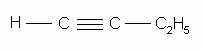 1-butyne |
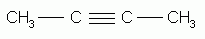 2-butyne |
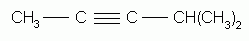 4-methyl-2-pentyne |
Other modules in this series:
[an error occurred while processing this directive]
