Organic Nomenclature - Alkanes
Other modules in this series:
Special thanks to Vicki Winterton for providing the outline for this learning module.
You may test your knowledge of knowledge of alkane nomenclature prior to studying this module, by using the Interactive Quiz on Alkanes
Carbon, because of its valence electrons, can form four bonds and hydrogen can form only one bond. The names of methane, ethane, propane, butane, and pentane are used for alkanes containing one, two, three, four, and five carbon atoms, respectively.
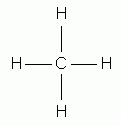 methane |
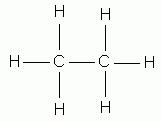 ethane |
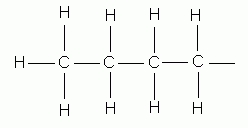
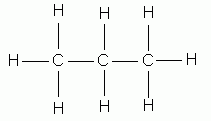 propane |
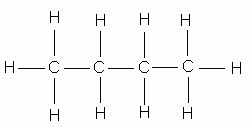 butane |
Except for the first four members of the family, the name is simply derived from the Greek (or Latin) prefix for the particular number of carbon atoms in the alkane; thus PENTane for five, HEXane for six, HEPtane for seven, OCTane for eight, NONane for nine, DECane for ten and so on. You should certainly know the first ten. The structures drawn above are called "normal" alkanes because they form in a straight line without side chains. These, as well as others, form the base of a multitude of organic compounds. From these normal alkanes, we derive the names of certain groups that constantly appear as structural units of organic molecules. For instance, chloromethane, CH3Cl, is also known as methyl chloride. The CH3- group is called "methyl" wherever it appears. CH3Br is thus called methyl bromide, CH3I is called methyl iodide, and CH3OH is called methyl alcohol. In the same way, the C2H5 group is "ethyl"; C3H7 - is propyl; C4H9 is butyl, and so on. The . . . means that something is attached at that point. Remember that carbon forms 4 bonds and hydrogen forms 1 bond, so when you see, for instance, C4H9 - , it must for instance, it must have a structure of:
With these things in mind, we will begin to name and draw structures for compounds using the system devised by IUPAC (International Union of Pure and Applied Chemistry). The rules devised by IUPAC for the alkanes are as follows:
- Select as the parent structure the longest continuous chain, and then consider the compound to have been derived from this structure by the replacement of hydrogen by various alkyl groups.
- Where necessary, as in the isomeric methyl pentanes, indicate by a number the carbon to which the branching alkyl group is attached. In numbering the parent carbon chain, start at whichever end results in the use of the lowest numbers; thus the naming of the following isomers:
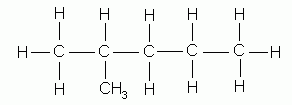 2-methylpentane |
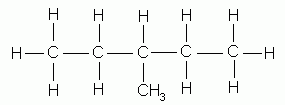 3-methylpentane |
Now, you might be wondering why there is no 1-methylpentane...
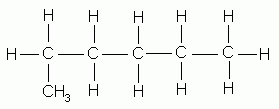
If you analyze the structure closely, you see that the added methyl group actually creates a longest chain that is now 6 carbon atoms in length. Thus, the structure above is correctly named hexane.
- If the alkyl group appears more than once as a side chain, indicate this by the prefix di-, tri-, tetra-, etc. These prefixes are used to show how many of these alkyl groups there are, and indicate by various numbers the positions of each group as shown below.
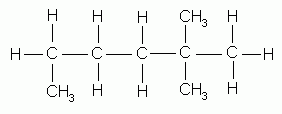
2,2-dimethylhexane
- If there are several different alkyl groups attached to the parent chain, name them in alphabetical order. The ethyl group is named before the methyl group because "e" (as in ethyl) comes alphabetically before "m" (as in methyl). The prefix does not contribute to the alphabetical order of the functional groups.
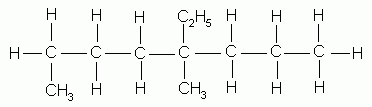
4-ethyl-4-methyloctane
- The prefix "iso-" is used to designate any alkyl group (of six carbons or less) that has a single one-carbon branch on the next-to-last carbon of a chain and has the point of attachment at the opposite end of the chain.
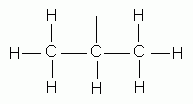 isopropyl group |
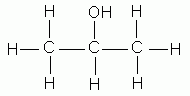 isopropyl alcohol |
Isopropyl alcohol could also be (correctly) named 2-propanol, denoting the presence of the alcohol functional group (OH-) attached to the second carbon in the 3-carbon propane chain.
Isomers
Different compounds that have the same molecular formula are called isomers. They contain the same numbers of the same kinds of atoms, but the atoms are attached to one another in different ways. Isomers are different compounds because they have different molecular structures. For instance, the two isomers of C4H10 are:
 butane |
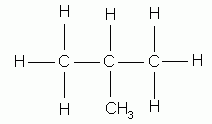 2-methyl propane |
As you can see, structural formulas can take up a lot of space when drawn out. In order to save space, structural formulas will often be written out in one line. In fact, I have already taken the liberty of using some shorthand in writing several of the alkyl groups as CH3 . and C2H5- . This is commonly done even when writing complete structural formulas, so to the difficulty of confining the bond lines to two dimensions. When you want to indicate that a chain is meant to be a side chain, it is written in parentheses.
Ready to practice? Try my Review of Alkane Nomenclature before moving on with the following modules.
Other modules in this series:
